Modern materials can be divided into four categories: metals, polymers, ceramics and composite materials. Despite the rapid development of macromolecule materials, steel is still the most widely used and most important material in the current engineering technology. What factors determine the dominant position of steel materials? Now let’s introduce it in detail.
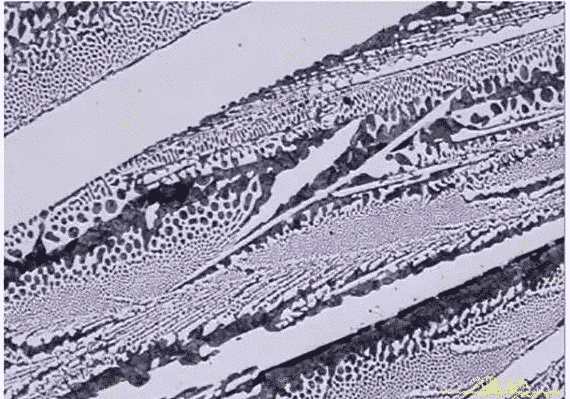
Iron and steel are extracted from iron ore, rich in sources and low in price. Iron and steel, also known as iron-carbon alloy, is an alloy composed of iron (Fe) and carbon (C), silicon (Si), manganese (Mn), phosphorus (P), sulfur (S) and other small elements (Cr, V, etc.). Various metallographic structures can be obtained by adjusting the content of various elements in steel and heat treatment process (four firings: quenching, annealing, tempering, normalizing), so that steel has different physical properties. The structure observed under metallographic microscope is called metallographic structure of steel after sampling, grinding, polishing and etching with a specific corrosive agent. The secrets of steel materials are hidden in these structures.
In Fe-Fe3C system, iron-carbon alloys with different compositions can be prepared. Their equilibrium structures are different at different temperatures, but they are composed of several basic phases (ferrite F, austenite A and cementite Fe3C). These basic phases are combined in the form of mechanical mixtures, forming a rich and colorful metallographic structure in steel. There are eight common metallographic structures:
I. Ferrite
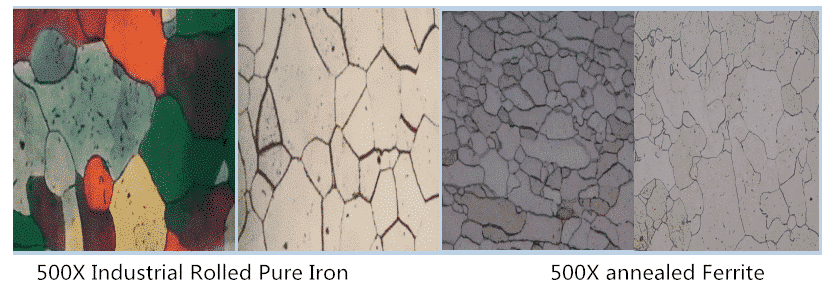
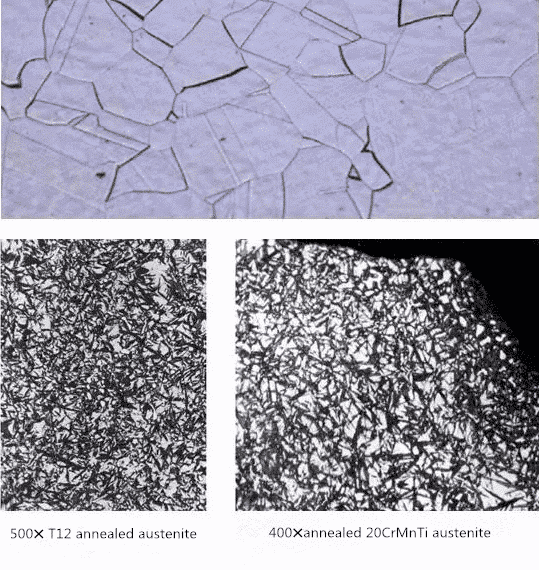
The interstitial solid solution formed by dissolving carbon in the interstitial of a-Fe lattice is called ferrite, which belongs to BCC Structure and is equiaxed polygonal grain distribution, which is expressed by symbol F. Its structure and properties are similar to pure iron. It has good plasticity and toughness, but its strength and hardness are lower (30-100 HB). In alloy steel, it is a solid solution of carbon and alloy elements in alpha-Fe. The solubility of carbon in alpha-Fe is very low. At AC1 temperature, the maximum solubility of carbon is 0.0218%, but with the decrease of temperature, the solubility decreases to 0.0084%. Therefore, the third cementite appears at the ferrite grain boundary under slow cooling condition. With the increase of carbon content in steel, the number of ferrite decreases and the number of pearlite increases. At this time, the ferrite is network and crescent.
Ⅱ.Austenite
The interstitial solid solution formed by the dissolution of carbon in the interstitial space of the gamma-Fe lattice is called austenite. It has a face-centered cubic structure and is a high temperature phase, which is represented by symbol A. Austenite has a maximum solubility of 2.11% C at 1148 C and solid solution of 0.77% C at 727 C. Its strength and hardness are higher than that of ferrite, its plasticity and toughness are good, and it is non-magnetic. Its specific mechanical properties are related to carbon content and grain size, generally 170-220 HBS, = 40-50%. TRIP steel is a steel developed on the basis of good plasticity and flexibility of austenite. The strain-induced transformation and transformation-induced plasticity of retained austenite are used to improve the plasticity of steel plate and the formability of steel plate. Austenite in carbon or alloy structural steels transforms into other phases during cooling. Only after carburizing and high temperature quenching of high carbon steels and carburized steels can austenite remain in martensite gap, and its metallographic structure is white because it is not easy to be eroded.
Ⅲ. Cementite
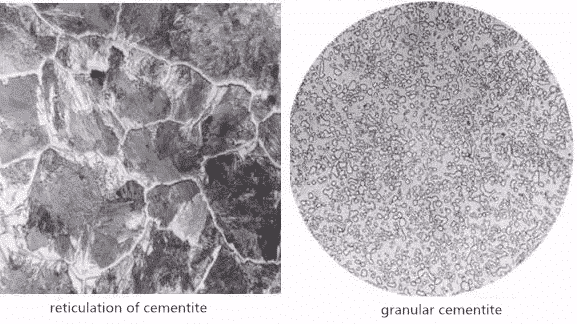
Cementite is a metal compound synthesized by a certain proportion of carbon and iron. The molecule formula Fe3C shows that its carbon content is 6.69%, and (Fe, M) 3C is formed in the alloy. The cementite is hard and brittle, its plasticity and impact toughness are almost zero, its brittleness is very high and its hardness is 800HB. In iron and steel, the distribution is usually network, semi-network, flake, needle-flake and granular.
IV. Pearlite
Pearlite is a mechanical mixture of ferrite and cementite, expressed in symbol P. Its mechanical properties are between ferrite and cementite, with high strength, moderate hardness and certain plasticity. Pearlite is a product of eutectoid transformation in steel. Its morphology is that ferrite and cementite are arranged in layers like fingerprints. According to the distribution pattern of carbides, it can be divided into two types: flake pearlite and spherical pearlite.

a. Flake pearlite: It can be divided into three types: thick flake, medium flake and fine flake.
b. Spherical pearlite: obtained by spheroidizing annealing, the cementite is spheroidized and distributed on the ferrite matrix. the size of cementite spheroids depends on the spheroidizing annealing process, especially the cooling rate. Spherical pearlite can be divided into four types: coarse spherical, spherical, fine spherical and punctate.
V. Bainite
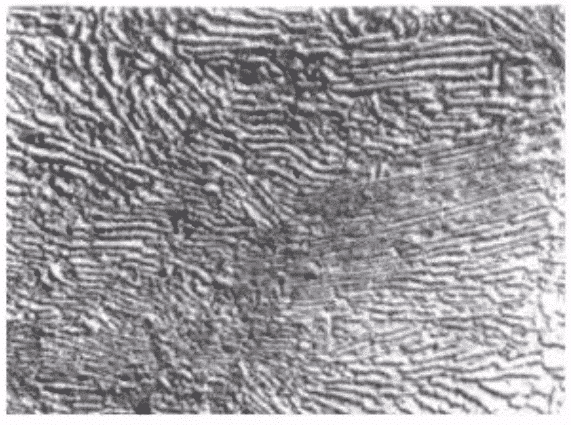
Bainite is the product of transformation of austenite below pearlite transformation zone and above MS point in medium temperature zone. Bainite is a mechanical mixture of ferrite and cementite, a structure between pearlite and martensite, expressed in symbol B. According to the formation temperature, it can be divided into granular bainite, upper bainite (upper B) and lower bainite (lower B). Granular bainite has low strength but good toughness. lower bainite has both high strength and good toughness. granular bainite has the worst toughness. Bainite morphology is changeable. According to its shape characteristics, bainite can be divided into three types: feather, needle and granular.
a. Upper bainite:
Upper bainite is characterized by the parallel arrangement of strip ferrite, with fine strip (or short rod) cementite parallel to the ferrite needle axis, feathery.
b. Lower bainite:
fine needle flake, with certain orientation, more vulnerable to erosion than quenched martensite, very similar to tempered martensite, very difficult to distinguish under light microscope, easy to distinguish under electron microscope. carbide precipitates in acicular ferrite, and its alignment orientation is 55-60 degrees with the long axis of ferrite sheet, lower bainite does not contain twins, there are more dislocations.

c. Granular bainite:
Ferrite with polygonal shape and many irregular island-like structures. When the austenite of steel is cooled to a little higher than the forming temperature of upper bainite, some carbon atoms of precipitated ferrite migrate from ferrite to austenite through ferrite/austenite phase boundary, which makes austenite unevenly rich in carbon, thus restraining the transformation from austenite to ferrite. These austenite regions are generally island-like, granular or strip-like, distributed on ferrite matrix. During continuous cooling, according to the composition of austenite and cooling conditions, the austenite in grain bails can undergo the following changes.
(i) Decomposition into ferrite and carbide in whole or in part. Under the electron microscope, granular, rod or small block carbides with dispersive multidirectional distribution can be seen.
(ii) partial transformation into martensite, which is fully yellow under light microscope.
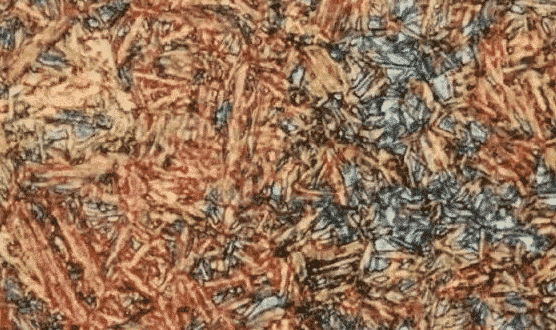
(iii) still retains carbon-rich austenite.
Granular carbides are distributed on the ferrite matrix of granular bainite (the island structure was originally carbon-rich austenite, which was decomposed into ferrite and carbide when cooled, or transformed into martensite or remained carbon-rich austenite particles). Feather bainite, ferrite matrix, strip carbide precipitated at the margin of ferrite sheet. Lower bainite, acicular ferrite with small flake carbide, flake carbide in the ferrite of the long axis is roughly 55 ~ 60 degrees angle.
VI. WEISHER’S TISSUE
Widmanstatten structure is a kind of superheated structure, which consists of ferrite needles intersecting each other about 60 degrees and embedded in the matrix of steel. Coarse Widmanstatten structure decreases the plasticity and toughness of steel and increases its brittleness. In hypoeutectoid steel, coarse grains are formed by overheating and precipitate rapidly when cooling. Therefore, in addition to the network precipitation along the austenite grain boundary, some ferrites are formed from grain boundary to grain in accordance with shear mechanism and separately precipitated into needles. The structure of this distribution is called Widmanstatten structure. When superheated supereutectoid steel is cooled, the cementite also extends from grain boundary to grain and forms Widmanstatten structure.

Ⅶ.Martensite

The supersaturated solid solution of carbon in alpha-Fe is called martensite. Martensite has high strength and hardness, but its plasticity is poor, almost zero. It can not bear impact load expressed by symbol M. Martensite is the product of rapid cooling of undercooled austenite and transformation of shear mode between MS and Mf points. At this time, carbon (and alloying elements) can not diffuse in time, only from the lattice (face center) of gamma-Fe to the lattice (body center) of alpha-Fe, that is, the solid solution (austenite) of carbon in gamma-Fe to the solid solution of carbon in alpha-Fe. Therefore, martensite transformation is based on the metallographic characteristics of martensite, which can be divided into lath martensite (low carbon) and acicular martensite.

a. lath martensite:
also known as low carbon martensite. Fine martensite strips of roughly the same size are aligned in parallel to form martensite bundles or martensite domains. the orientation difference between domains and domains is large, and several domains with different orientations can be formed in a primitive austenite grain. Because of the high temperature of lath martensite formation, the phenomenon of self-tempering will inevitably occur in the cooling process, and carbides will precipitate in the formed martensite, so it is vulnerable to erosion and darkening.
b. acicular martensite:
also known as flake martensite or high carbon martensite, its basic characteristics are: the first martensite sheet formed in an austenite grain is relatively large, often throughout the whole grain, the austenite grain is divided, so that the size of martensite formed later is limited, so the size of flake martensite varies, irregular distribution. The acicular martensite is formed in a certain direction. There is a middle ridge in the martensite needle. The higher the carbon content, the more obvious the martensite is. At the same time, there is white retained austenite between the martensite.

c. The martensite formed after quenching can also form three special metallographic structures after tempering:
(i) Tempered martensite:
the composite of sheet martensite formed during quenching (with a crystal structure of tetragonal body center) which is decomposed in the first stage of tempering, in which carbon is desolved in the form of transition carbides, and extremely fine transition carbide sheets dispersed in the solid solution matrix (whose crystal structure has changed into body-centered cube) (the interface with the matrix is a coherent interface) Phase structure. this kind of structure can not distinguish its internal structure even when magnified to the maximum magnification under metallographic (optical) microscope, only can see that its whole structure is black needle (the shape of black needle is basically the same as that of white needle formed during quenching). This kind of black needle is called “tempered martensite”.
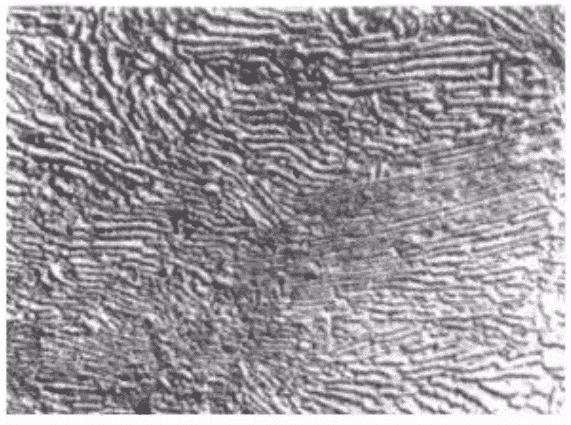
(ii) Tempered troostite:
product of quenched martensite tempered at medium temperature, characterized by gradual disappearance of needle shape of martensite, but still vaguely visible (chromium-containing alloy steel, its alloy ferrite recrystallization temperature is higher, so it still retains needle shape), precipitated carbides are small, difficult to distinguish under light microscope, carbide particles can only be seen under electron microscope, pole Susceptible to erosion and blackening of tissues. If tempering temperature is higher or retained for a longer time, the needles will be white. At this time, the carbides will be concentrated on the edge of the needles, and the hardness of the steel will be slightly lower and the strength will decrease.
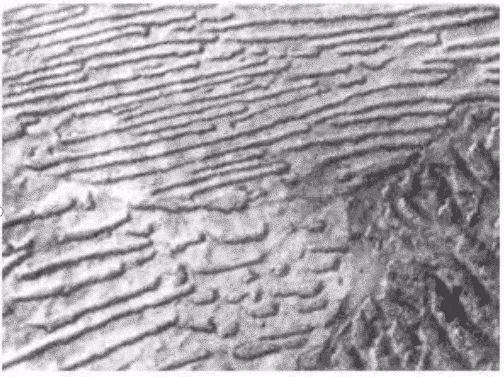
(iii) tempered sorbite:
product of quenched martensite tempered at high temperature. Its characteristics are: fine granular carbides are distributed on the sorbite matrix, which can be distinguished clearly under the light microscope. This kind of structure, also known as conditioned structure, has a good combination of strength and toughness. The finer the fine carbides on ferrite, the higher the hardness and strength, and the worse the toughness. on the contrary, the lower the hardness and strength, and the higher the toughness.
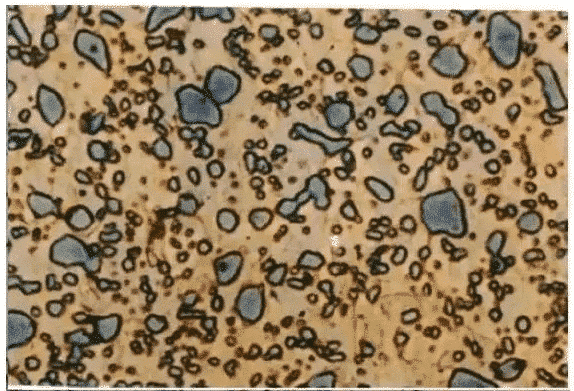
Ⅷ.Ledeburite

The eutectic mixtures in FERROCARBON alloys, i.e. liquid FERROCARBON alloys with a mass fraction of carbon (carbon content) of 4.3%, are called ledeburite when the mechanical mixtures of austenite and cementite crystallize simultaneously from the liquid at 1480 degrees Celsius. Since austenite transforms into pearlite at 727 C, ledeburite is composed of pearlite and cementite at room temperature. In order to distinguish the ledeburite above 727 C is called high-temperature ledeburite (L d), and the ledeburite below 727 C is called low-temperature ledeburite (L’d). The properties of ledeburite are similar to those of cementite with high hardness and poor plasticity.