The article introduces a new method called “amorphous crystallization + reactive transformation” for preparing dense and uniformly structured nano carbides. The formation and evolution process of nanocrystalline multiphase structures are systematically studied. A series of heating and quenching experiments are designed to investigate the nanocrystalline nucleation and initial phase transformation of multicomponent amorphous powders. The parallel processes of grain growth and interface formation are explored, revealing their micro-mechanisms.
Furthermore, the study examines the influence of nanoscale structures and interface characteristics on the mechanical properties of the prepared nanocrystalline composite materials. It emphasizes the significant role and underlying mechanisms of interface coherency in refining the microstructure of carbides during the loading process. The insights provided by this research offer a new perspective on achieving high overall mechanical performance in nanocrystalline composite materials through the utilization of specific interface relationships.
Research Objective
carbide materials are generally hard and brittle, with very limited plasticity, primarily due to the intrinsic properties of the covalent bonds in the matrix ceramic phase. Due to the inherent characteristics of covalent crystals, strengthening strategies commonly used in metallic materials such as solid solution strengthening, dislocation (deformation) strengthening, and precipitation strengthening are difficult to apply to ceramics and ceramic-based composites. Fine-grain strengthening is often employed to enhance the strength of ceramic materials. However, as grain size decreases, especially when it reaches the nanometer scale, the hardness of ceramic grains increases, but the toughness decreases significantly. Therefore, finding an optimal balance between the hardness and toughness of ceramic materials has always been a challenging problem in the field.
WC-Co carbide is a typical representative of ceramic/metal composites. In ultrafine and nano carbides, as the grain size decreases, the volume fraction of interfaces increases rapidly. Hence, interface characteristics play an increasingly important role in the mechanical behavior of composite materials with refined grain structures. In nano carbide composites, refining ceramic grain size and modulating interface bonding characteristics can effectively improve their overall mechanical performance.
Research Methods
The Co-W-C amorphous powder mixture prepared using amorphous Co2W4C composite powder and carbon black first forms the Co3W3C phase as the temperature rises. Subsequently, the WC phase is formed, and finally, the Co phase is formed. Through the study of the growth process after the crystallization of WC, the formation and growth mechanism of the hard phase originating from the amorphous matrix in the WC-Co composite material are revealed.
In the initial stage of WC nucleation, WC crystals exhibit regular shapes on the observation plane and have equidistant edges along the [100] and [0001] directions. (0001) basal planes and (100) prism planes grow alternately through a “step” mechanism, where the step thickness is approximately 1 to 2 atomic layers. The growth of WC crystals along the [0001] and [100] directions is essentially isotropic, resulting in the formation of equiaxed (110) crystal faces.
Research Results
In the “amorphous crystallization + reactive transformation” new method described in this article, the WC and Co phases are formed through the crystallization and carbide process of the amorphous matrix. During this process, coherent or semi-coherent WC/Co phase boundaries (PB) are formed in the nanocrystalline WC-Co composite material. As the WC grains grow, S2 WC/WC grain boundaries (GB) may form between nanocrystalline grains. Simultaneously, PB and GB serve as diffusion and migration channels for atoms, promoting grain growth and phase evolution. Therefore, compared to traditional carbides prepared by sintering a mixture of WC and Co powders, the nano carbide fabricated using this method exhibits a significantly increased proportion of coherent interfaces.

Microstructural evolution of amorphous Co-W-C powder at different temperatures in Figure 1: (a) At room temperature, disordered amorphous structure; (b) at 550 °C, preferential nucleation of Co3W3C nanocrystals; (c) at 750 °C, coexistence of amorphous matrix, Co3W3C nanophase, and a small amount of WC nanocrystals; (d) at 800 °C, nearing completion of crystallization; (e) at 900 °C, fully crystallized nanocrystalline structure; (f) at 1150 °C, dense nanocrystalline structure with only WC and Co phases.
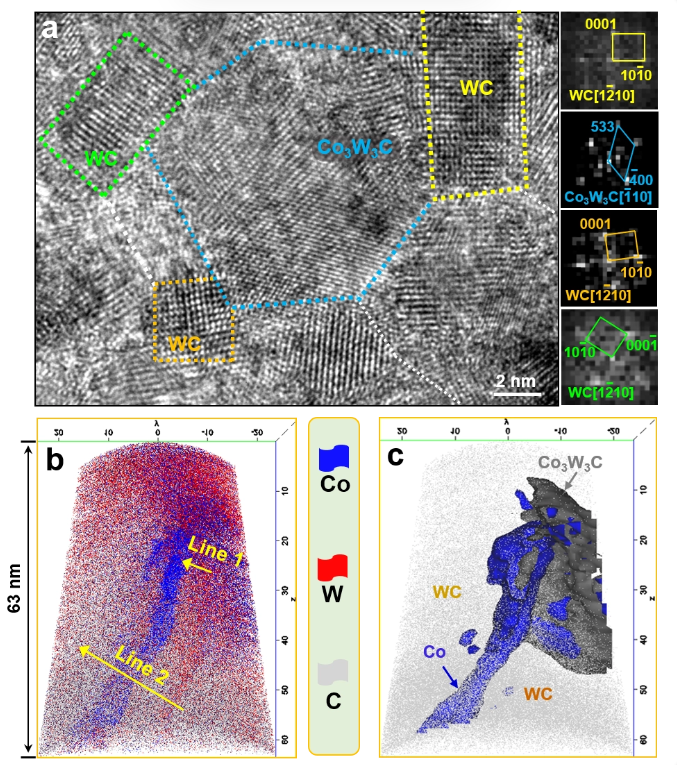
Figure 2: Phase and composition analysis of samples after heating-quenching at 800 °C and 900 °C:
(a) High-resolution transmission electron microscopy (HRTEM) image and phase analysis of the amorphous powder heated at 800 °C.
(b) Atom probe tomography (APT) analysis of the distribution of W, Co, and C elements in the sample heated at 900 °C.
(c) Phase configuration of the localized region determined by the composition analysis in (b).
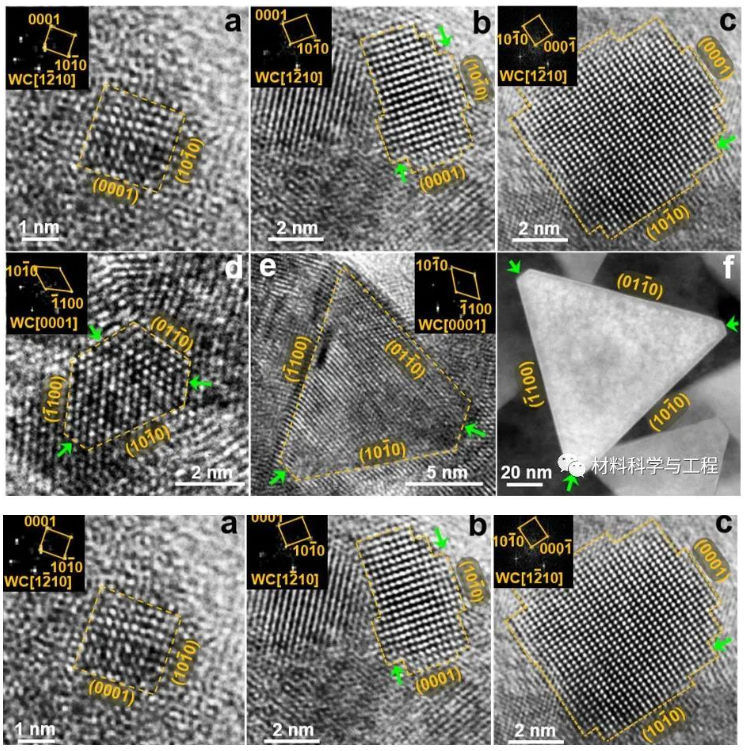
Figure 3: Observation of WC crystal nucleation and growth from the amorphous matrix on typical characteristic crystal planes:
(a-c) Formation and growth characteristics of the (110) crystal plane of WC, corresponding to the heating-quenching conditions at 750 °C, 800 °C, and 850 °C, respectively.
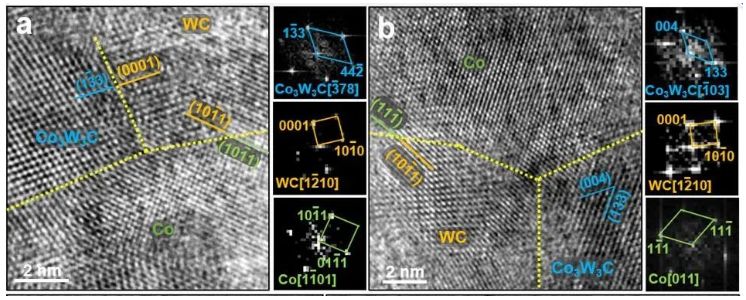
Figure 4: Characteristics of grain boundaries and phase boundaries in the sample heated and quenched at 850 °C:
(a) Co3W3C/WC and WC/hcp-Co coherent phase boundaries.
(b) WC/fcc-Co coherent phase boundary.
(c) Coalescence and growth of WC with the same orientation.
(d) Coalescence and growth of WC grains with Σ2 grain boundaries.
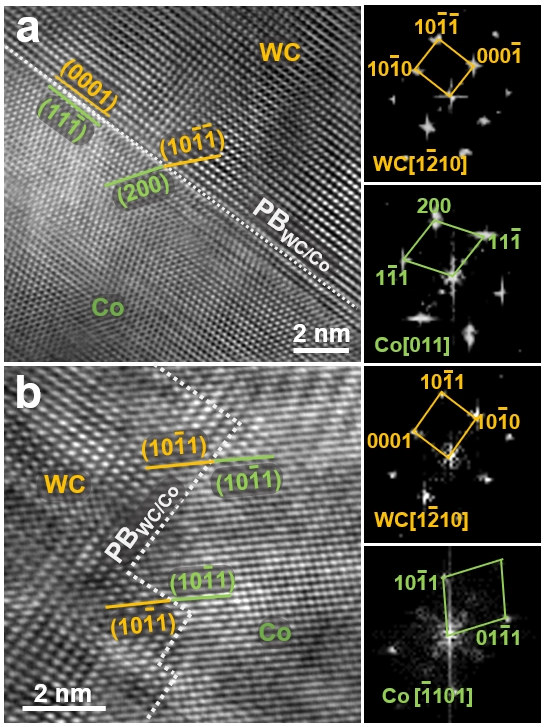
Figure 5: In the fully densified nano carbide sintered at 1150 °C, containing only WC and Co phases, examples of coherent interfaces are shown:
(a) WC/fcc-Co phase boundary.
(b) WC/hcp-Co phase boundary.
Wniosek
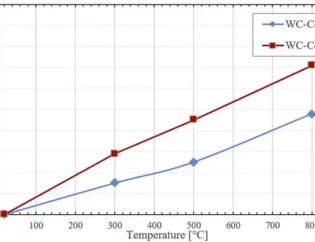
The nano carbide prepared in this study exhibits both high hardness (1775±23 kgf/mm²) and high fracture toughness (15.20±0.13 MPa·m^1/2), achieving a comprehensive mechanical performance at the forefront of similar materials in the literature. The significant increase in the proportion of special coherent interfaces in the nanocrystalline carbide, prepared through the crystallization and in-situ reaction of amorphous powder mixture and subsequent sintering densification, promotes the transfer of stress across phase boundaries between the hard phase and tough metallic phase, ensuring continuity in the deformation of the metallic phase and ceramic phase. Consequently, the interfaces in the carbide prepared by this method allow for uniform strain distribution from the metallic to the ceramic phase, avoiding stress concentration. As a result, the material exhibits not only high hardness due to nanoscaling but also significantly improved fracture toughness, thanks to the presence of a high proportion of special coherent interfaces.
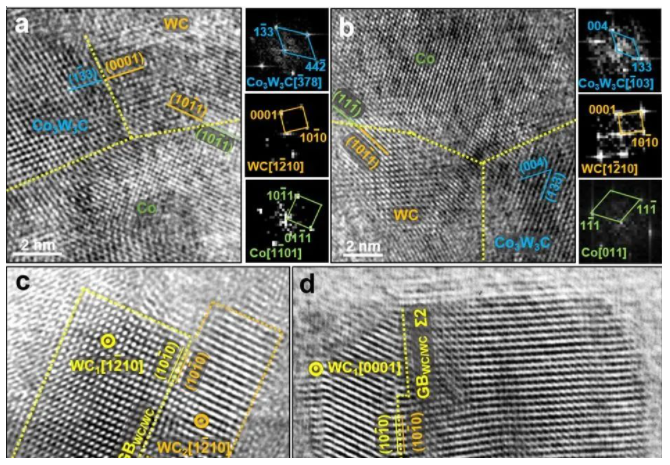
Figure 6: Microstructure of the prepared nanocrystalline carbide and the continuous deformation mechanism of the metallic phase and ceramic phase at coherent interfaces:
(a) Morphology of WC and Co grains in the material after compression.
(b) Dislocations crossing through the semi-coherent WC/Co phase boundary within Co and WC grains.
(c) Schematic illustration of the dislocation motion between adjacent phases and continuous deformation across the WC/Co phase boundary.
(d) Deformation incompatibility and discontinuity at the incoherent WC/Co phase boundary.